A promising drug candidate passes every 2D cell culture test. The data looks perfect. Then it fails in clinical trials because it behaves completely differently in actual human tissue. This scenario plays out in pharmaceutical labs every day. The problem isn’t the science or the scientists. It’s the fundamental limitation of using 2D cell models to determine drug efficacy and toxicity when those models don’t adequately address the complexity of real world 3D tissues.
Ex vivo models and 3D samples like tumouroids and clinical biopsies are more biologically relevant than traditional 2D cell assays. But moving beyond traditional cell models requires tools designed specifically for cellular phenotyping of ex vivo samples.
Every drug discovery researcher knows the frustration. Your 2D cell assay is optimised. The results are reproducible. But then the compound behaves differently in more complex models or clinical settings.
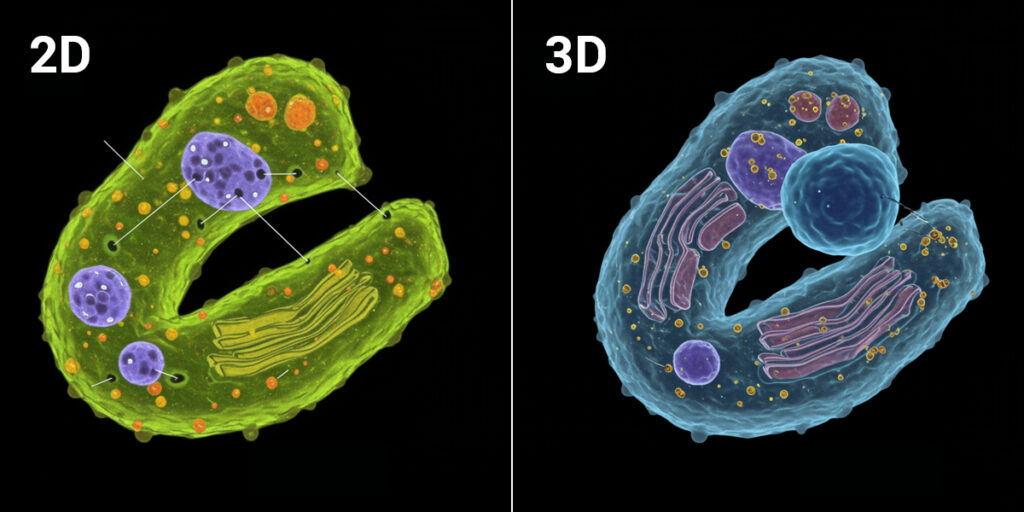
The quiet truth heard across pharmaceutical labs: using 2D cell models to determine drug efficacy and toxicity does not adequately address the complexity of real world 3D tissues. Cells growing in a monolayer on plastic experience conditions that simply don’t exist in actual organs or tumours. When researchers try to move beyond 2D cultures, they face a critical challenge: lack of tools for cellular phenotyping of ex vivo samples. Clinical biopsies are precious material that can’t be wasted on methods not designed for them. Tumouroids and organoids have thickness and complexity that traditional tools struggle to analyse. The very characteristics that make these models valuable also make them harder to work with.
The result? Many labs continue using 2D assays not because they provide better data, but because the alternatives seem too difficult. Experiments stall. Toxicity data remains unclear. The gap between laboratory findings and clinical outcomes persists.This isn’t a problem researchers can solve through better technique alone. It requires instrumentation designed for the realities of three-dimensional biology.
Biologically relevant 3D cell models provide more useful data than traditional 2D cultures. But why does dimension matter so much?
Ex vivo models help researchers gain insight into a drug’s mechanism of action and safety within a more physiologically relevant context than cell cultures. When you test a drug on cells growing flat on plastic, you’re studying biology that doesn’t exist in patients. When you test the same drug in ex vivo samples like clinical biopsies or tumouroids, you’re studying biology that actually resembles the tissue the drug will encounter.
3D samples like tumouroids and ex vivo clinical biopsies are more biologically relevant than traditional 2D cell assays because they retain structural complexity, cell-to-cell interactions and tissue architecture that influence how drugs actually work. A compound that appears effective in a monolayer might fail to penetrate a 3D structure. A drug that seems safe in 2D might trigger unexpected responses when cells interact in three dimensions.This isn’t just about making experiments more complicated. It’s about making data more predictive. The goal of drug development is to understand how compounds will behave in patients. Ex vivo systems move researchers closer to that reality. The challenge has been measuring these complex samples reliably. That’s where tool selection becomes critical.
Ex vivo workflows can actually improve efficiency when paired with the right tools, even though they appear more complex at first glance. The key is choosing instruments designed specifically for the samples you’re studying rather than trying to adapt tools built for 2D cultures. When you lack tools for cellular phenotyping of ex vivo samples, every experiment becomes trial and error. You spend time troubleshooting methods that were never designed for thick, irregular, three-dimensional samples. You waste precious clinical material on approaches that can’t capture the biology you need to see.
The right tools eliminate this friction. Instruments designed for ex vivo analysis accommodate sample complexity from the start. They’re built to handle the thickness, irregularity and physiological relevance that make 3D models valuable. This reduces the experimental iterations needed to generate meaningful data. Efficiency isn’t about shortcuts. It’s about matching tool capabilities to sample reality so researchers spend less time fighting their instruments and more time understanding biology.
Analysing drug efficacy and toxicity in ex vivo samples requires understanding how drugs affect cellular function in samples that retain three-dimensional structure. Traditional methods often require destroying the very architecture that makes ex vivo models valuable. The Agilent Seahorse XF Flex Analyser is designed for the analysis of ex vivo samples so researchers can gain insight into a drug’s mechanism of action and safety within a more physiologically relevant context than cell cultures. The system works with clinical biopsies, tumouroids and organoids without requiring tissue dissociation.
This matters because ex vivo models help researchers gain insight into drug mechanism of action and safety within a more physiologically relevant context than 2D cell cultures. By analysing intact tissue samples, researchers can assess how drugs affect cellular metabolism in conditions that actually resemble patient biology. The Seahorse XF Flex addresses the lack of tools for cellular phenotyping of ex vivo samples by providing metabolic profiling capabilities specifically designed for these challenging sample types.
24-well metabolic analyzer, Seahorse XF Flex Analyzer | Agilent
Understanding cellular responses in ex vivo samples requires seeing into thick, complex structures. Traditional imaging approaches struggle with samples that extend beyond a few cell layers, forcing researchers to either section valuable material or accept surface-level data that misses critical biology.
The Agilent BioTek Cytation C10 Confocal Imaging Reader allows researchers to look deeper into thick sample biology with improved clarity and detail. The confocal imaging system addresses the lack of tools for cellular phenotyping of ex vivo samples by providing the depth resolution needed for three-dimensional structures.
This capability matters for drug development because biologically relevant 3D cell models provide more useful data when you can actually see what’s happening throughout the structure, not just on the surface. Cell migration, invasion and drug responses often vary by location within a tumouroid or tissue sample. Without appropriate imaging tools, researchers miss spatial information critical to understanding efficacy and toxicity.
The Cytation C10 enables cellular phenotyping of the ex vivo samples that provide physiologically relevant data for drug discovery.

Confocal High Content Imaging Microscope, BioTek Cytation C10 | Agilent
There’s a common mindset in drug discovery that researchers must accept the limitations of 2D models because ex vivo systems are too difficult. That working with clinical biopsies isn’t practical. That 3D cultures are too complex for routine use.
Chemetrix rejects this narrative.Researchers shouldn’t settle for models that don’t adequately address the complexity of real world 3D tissues. They shouldn’t compromise scientific rigour because tools weren’t designed for physiologically relevant samples. And they absolutely shouldn’t accept that predicting clinical outcomes requires choosing between feasibility and accuracy.
This is where partnership matters. Chemetrix doesn’t just supply instruments. We advocate for a scientific culture grounded in integrity, accuracy and respect for the complexity of living systems. When we say ex vivo models provide more useful data, we’re affirming that good science requires data that reflect real biology. The lack of tools for cellular phenotyping of ex vivo samples has been a barrier for too long. The Seahorse XF Flex and Cytation C10 aren’t just instruments. They’re commitments to removing barriers between researchers and the capabilities they need.
Chemetrix partners with researchers because they deserve solutions designed with biological reality in mind. Ex vivo systems that work reliably. Analysis that captures what matters. Support that helps labs implement physiologically relevant models confidently. This is how drug development advances: not through researchers heroically overcoming inadequate tools, but through systems that make biological relevance routine.

Drug development doesn’t have to rely on models that don’t adequately address the complexity of real world 3D tissues. Biologically relevant 3D cell models provide more useful data. But only when you have tools designed for cellular phenotyping of ex vivo samples.
Discover how the Agilent Seahorse XF Flex enables analysis of clinical biopsies and 3D cultures. Explore how the Agilent BioTek Cytation C10 allows researchers to look deeper into thick sample biology with improved clarity and detail.
Contact Chemetrix to discuss how ex vivo workflows can improve the predictive value of your drug discovery research. Better models lead to better medicines. With the right partnership and the right tools, your lab can generate the clinically relevant data that truly matters.